Transformation of chlorpyrifos and chlorpyrifos-methyl in prairie pothole pore waters†
Abstract
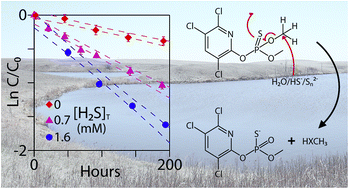
Non-point source pesticide pollution is a concern for wetlands in the prairie pothole region (PPR). Recent studies have demonstrated that reduced sulfur species (e.g., bisulfide and polysulfides) in PPR wetland pore waters directly undergo reactions with chloroacetanilide and dinitroaniline compounds. In this paper, the abiotic transformation of two organophosphate compounds, chlorpyrifos and chlorpyrifos-methyl, was studied in PPR wetland pore waters. Chlorpyrifos-methyl reacted significantly faster (up to 4 times) in pore water with reduced sulfur species relative to hydrolysis. No rate enhancement was observed in the transformation of chlorpyrifos in pore water with reduced sulfur species. The lack of reactivity was most likely caused by steric hindrance from the ethyl groups and partitioning to dissolved organic matter (DOM), thereby shielding chlorpyrifos from nucleophilic attack. Significant decreases in reaction rates were observed for chlorpyrifos in pore water with high concentrations of DOM. Rate enhancement due to other reactive species (e.g., organo-sulfur compounds) in pore water was minor for both compounds relative to the influence of bisulfide and DOM.
- This article is part of the themed collection: Editor’s Choice: Underappreciated Science

 Please wait while we load your content...
Please wait while we load your content...