A self-defense redox mediator for efficient lithium–O2 batteries†
Abstract
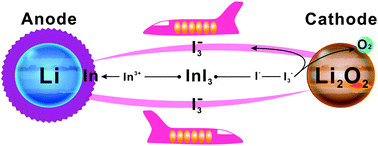
Redox mediators (RMs) have become focal points in rechargeable Li–O2 battery research to reduce overpotentials in oxygen evolution (charge) reactions. In this study, we found an evidence for the shuttle effect arising in dimethyl sulfoxide (DMSO) with a LiI RM through the visual observation of the diffusion of soluble I3− towards a Li anode where it reacted chemically to produce LiI, which can be only partly dissolved, leading to the loss of both the RM and electrical energy efficiency. Therefore, we proposed a self-defense redox mediator (SDRM) of InI3 to counter this problem. During charging, the In3+ is reduced electrochemically on the Li anode prior to Li+, forming a much stable indium layer to resist the synchronous attack by the soluble I3−. The pre-deposited indium layer can also reduce the growth of dendrites from the Li anode surface. As a result, the electrical energy efficiency and the cycling performance of the Li–O2 cells were improved significantly.

 Please wait while we load your content...
Please wait while we load your content...