Bulky α-diimine palladium complexes: highly efficient for direct C–H bond arylation of heteroarenes under aerobic conditions†
Abstract
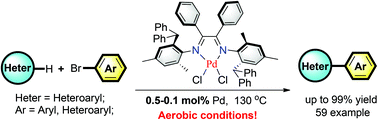
Through the strategy to enhance the bulkiness on both the backbone and the N-aryl moieties, we designed and synthesized a type of bulky α-diimine palladium complex (i.e., {[Ar–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(R)–C(R)
C(R)–C(R)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–Ar]PdCl2, (Ar = 2-benzhydryl-4,6-dimethylphenyl)}, C1, R = H; C2, R = An; C3, R = Ph). The structures of these palladium complexes were well characterized, while C1 and C3 were further characterized by X-ray diffraction. The catalytic performances of the precatalysts were screened for direct C–H bond arylation of heteroarenes. The bidentate N,N-palladium complex C3 with both a backbone and N-aryl bulkiness was found to be a highly efficient precatalyst under aerobic conditions. With a low palladium loading of 0.5–0.1 mol%, a variety of heteroarenes with challenging bulky steric aryl bromides as well as heteroaryl bromides are all applicable for this cross-coupling reaction.
N–Ar]PdCl2, (Ar = 2-benzhydryl-4,6-dimethylphenyl)}, C1, R = H; C2, R = An; C3, R = Ph). The structures of these palladium complexes were well characterized, while C1 and C3 were further characterized by X-ray diffraction. The catalytic performances of the precatalysts were screened for direct C–H bond arylation of heteroarenes. The bidentate N,N-palladium complex C3 with both a backbone and N-aryl bulkiness was found to be a highly efficient precatalyst under aerobic conditions. With a low palladium loading of 0.5–0.1 mol%, a variety of heteroarenes with challenging bulky steric aryl bromides as well as heteroaryl bromides are all applicable for this cross-coupling reaction.

 Please wait while we load your content...
Please wait while we load your content...