A cobalt(ii) iminoiodane complex and its scandium adduct: mechanistic promiscuity in hydrogen atom abstraction reactions†
Abstract
In addition to oxometal [Mn+![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O] and imidometal [Mn+
O] and imidometal [Mn+![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NR] units, transient metal–iodosylarene [M(n−2)+–O
NR] units, transient metal–iodosylarene [M(n−2)+–O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) IPh] and metal–iminoiodane [M(n−2)+–N(R)
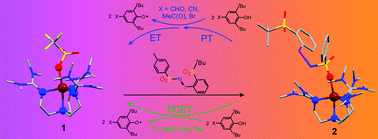
IPh] and metal–iminoiodane [M(n−2)+–N(R)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) IPh] adducts are often invoked as a possible “second oxidant” responsible for the oxo and imido group transfer reactivity. Although a few metal–iodosylarene adducts have been recently isolated and/or spectroscopically characterized, metal–iminoiodane adducts have remained elusive. Herein, we provide UV-Vis, EPR, NMR, XAS and DFT evidence supporting the formation of a metal–iminoiodane complex 2 and its scandium adduct 2-Sc. 2 and 2-Sc are reactive toward substrates in the hydrogen-atom and nitrene transfer reactions, which confirm their potential as active oxidants in metal-catalyzed oxidative transformations. Oxidation of para-substituted 2,6-di-tert-butylphenols by 2 and 2-Sc can occur by both coupled and uncoupled proton and electron transfer mechanisms; the exact mechanism depends on the nature of the para substituent.
IPh] adducts are often invoked as a possible “second oxidant” responsible for the oxo and imido group transfer reactivity. Although a few metal–iodosylarene adducts have been recently isolated and/or spectroscopically characterized, metal–iminoiodane adducts have remained elusive. Herein, we provide UV-Vis, EPR, NMR, XAS and DFT evidence supporting the formation of a metal–iminoiodane complex 2 and its scandium adduct 2-Sc. 2 and 2-Sc are reactive toward substrates in the hydrogen-atom and nitrene transfer reactions, which confirm their potential as active oxidants in metal-catalyzed oxidative transformations. Oxidation of para-substituted 2,6-di-tert-butylphenols by 2 and 2-Sc can occur by both coupled and uncoupled proton and electron transfer mechanisms; the exact mechanism depends on the nature of the para substituent.
- This article is part of the themed collection: Small Molecule Activation

 Please wait while we load your content...
Please wait while we load your content...