Antiproliferative activity of ruthenium(ii) arene complexes with mono- and bidentate pyridine-based ligands†
Abstract
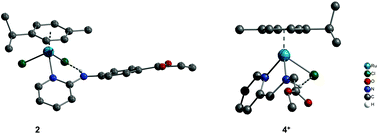
A series of RuII arene complexes of mono- and bidentate N-donor ligands with carboxyl or ester groups and chlorido ancillary ligands were synthesised and structurally characterised. The complexes have a distorted tetrahedral piano-stool geometry. The binding interaction was studied with calf thymus DNA (CT-DNA) by absorption titration, viscosity measurement, thermal melting, circular dichroism, ethidium bromide displacement assay and DNA cleavage of plasmid DNA (pBR322), investigated by gel electrophoresis. The dichlorido complexes bind covalently to DNA in the dark, similar to cisplatin, while the monochlorido complexes bind covalently on irradiation, similar to cisplatin analogues. The compounds are selectively cytotoxic against several tumour cell lines and show specific nonlinear correlation between dose and activity. This phenomenon is closely related to their potential to act preferentially as inhibitors of cell division.
- This article is part of the themed collection: Metallodrugs: Activation, Targeting, and Delivery

 Please wait while we load your content...
Please wait while we load your content...