Construction of magnet-type coordination polymers using high-spin {Ni4}-citrate cubane as secondary building units†
Abstract
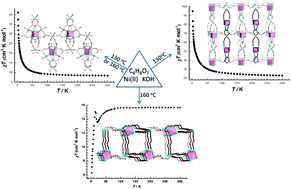
Three potassium(I)–nickel(II)–citrate coordination polymers, [K4Ni6(cit)4(H2O)8]n (1), [K14Ni17(cit)12(H2O)33]n·10nH2O (2) and [K8Ni12(cit)8(H2O)15]n·2nH2O (3), have been self-assembled in a solvothermal synthesis. Interestingly, these three polymers share the common {Ni4(cit)4}8− cubane ({Ni4}-cit-cub) secondary building units. The diverse ways of linking the {Ni4}-cit-cubs and additional isolated octahedral Ni(II) ions lead to disparate magnetic exchange-coupling interactions, namely ferromagnetic for 1 and 2 and antiferromagnetic for 3. More importantly, the weak ferromagnetic interactions do not lead to long-range magnetic ordering above 2 K in 1 or 2, whereas the strong antiferromagnetic interaction in 3 leads to uncompensated magnetic moment due to the non-collinear alignment of the spins. Further magnetic characterization confirms the coexistence of spin-canted antiferromagnetism and spin glass behaviour in 3.

 Please wait while we load your content...
Please wait while we load your content...