The contrasting catalytic efficiency and cancer cell antiproliferative activity of stereoselective organoruthenium transfer hydrogenation catalysts†
Abstract
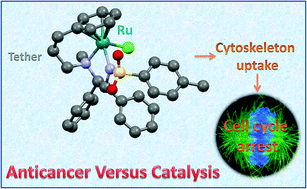
The rapidly growing area of catalytic ruthenium chemistry has provided new complexes with potential as organometallic anticancer agents with novel mechanisms of action. Here we report the anticancer activity of four neutral organometallic RuII arene N-tosyl-1,2-diphenylethane-1,2-diamine (TsDPEN) tethered transfer hydrogenation catalysts. The enantiomers (R,R)-[Ru(η6-C6H5(CH2)3-TsDPEN-N-Me)Cl] (8) and (S,S)-[Ru(η6-C6H5(CH2)3-TsDPEN-N-Me)Cl] (8a) exhibited higher potency than cisplatin against A2780 human ovarian cancer cells. When the N-methyl was replaced by N–H, i.e. to give (R,R)-[Ru(η6-Ph(CH2)3-TsDPEN-NH)Cl] (7) and (S,S)-[Ru(η6-Ph(CH2)3-TsDPEN-NH)Cl] (7a), respectively, anticancer activity decreased >5-fold. Their antiproliferative activity appears to be linked to their ability to accumulate in cells, and their mechanism of action might involve inhibition of tubulin polymerisation. This appears to be the first report of the potent anticancer activity of tethered RuII arene complexes, and the structure–activity relationship suggests that the N-methyl substituents are important for potency. In the National Cancer Institute 60-cancer-cell-line screen, complexes 8 and 8a exhibited higher activity than cisplatin towards a broad range of cancer cell lines. Intriguingly, in contrast to their potent anticancer properties, complexes 8/8a are poor catalysts for asymmetric transfer hydrogenation, whereas complexes 7/7a are effective asymmetric hydrogenation catalysts.

 Please wait while we load your content...
Please wait while we load your content...