Electrochemical deposition of highly-conducting metal dithiolene films†
Abstract
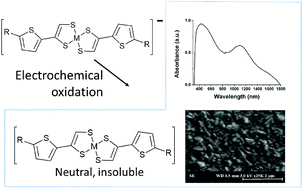
Electrochemical deposition has been used to prepare a thin film of neutral 4′,4-(3-alkyl)-thiophene-5′,5-hydogen-nickel and copper dithiolenes (Ni–C2, Cu–C2). The application of molecular electrodeposition provides a means to solution process molecular semiconductors of poor solubility, which results from the strong intermolecular interaction required for charge transport. Both Ni–C2 and Cu–C2 form continuous thin films that show intense NIR absorptions, extending to 1800 nm and 2000 nm respectively giving evidence for the strong intermolecular interactions in the solid state. Both films are highly conducting and temperature dependence of resistance gave an activation energy of 0.42 eV and 0.072 eV respectively, with the near-metallic behaviour of Cu–C2 attributed to the additional presence of an unpaired electron.

 Please wait while we load your content...
Please wait while we load your content...