Thermodynamic description of Tc(iv) solubility and hydrolysis in dilute to concentrated NaCl, MgCl2 and CaCl2 solutions†
Abstract
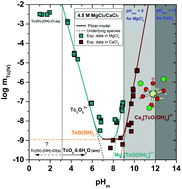
We present the first systematic investigation of Tc(IV) solubility, hydrolysis and speciation in dilute to concentrated NaCl, MgCl2 and CaCl2 systems, and comprehensive thermodynamic and activity models for the system Tc4+–H+–Na+–Mg2+–Ca2+–OH−–Cl−–H2O using both SIT and Pitzer approaches. The results are advancing the fundamental scientific understanding of Tc(IV) solution chemistry and are highly relevant in the applied context of nuclear waste disposal. The solubility of Tc(IV) was investigated in carbonate-free NaCl–NaOH (0.1–5.0 M), MgCl2 (0.25–4.5 M) and CaCl2 (0.25–4.5 M) solutions within 2 ≤ pHm ≤ 14.5. Undersaturation solubility experiments were performed under an Ar atmosphere at T = 22 ± 2 °C. Strongly reducing conditions (pe + pHm ≤ 2) were imposed with Na2S2O4, SnCl2 and Fe powder to stabilize technetium in the +IV redox state. The predominance of Tc(IV) in the aqueous phase was confirmed by solvent extraction and XANES/EXAFS spectroscopy. Solid phase characterization was accomplished after attaining thermodynamic equilibrium using XRD, SEM–EDS, XANES/EXAFS, TG–DTA and quantitative chemical analysis, and indicated that TcO2·0.6H2O(s) exerts solubility-control in all evaluated systems. The definition of the polyatomic Tc3O52+ species instead of TcO2+ is favoured under acidic conditions, consistently with slope analysis (mTcvs. pHm) of the solubility data gained in this work and spectroscopic evidence previously reported in the literature. The additional formation of Tc(IV)–OH/O–Cl aqueous species in concentrated chloride media ([Cl−] = 9 M) and pHm ≤ 4 is suggested by solubility and EXAFS data. The pH-independent behaviour of the solubility observed under weakly acidic to weakly alkaline pHm conditions can be explained with the equilibrium reaction TcO2·0.6H2O(s) + 0.4H2O(l) ⇔ TcO(OH)2(aq). Solubility data determined in dilute NaCl systems with pHm ≥ 11 follow a well-defined slope of +1, consistent with the predominance of TcO(OH)3− previously selected by NEA–TDB. In concentrated MgCl2 and CaCl2 solutions with pHm ≥ 8, the formation of the ternary Mg3[TcO(OH)5]3+ and Ca3[TcO(OH)5]3+ species is proposed based on the slope analysis of the solubility data, model calculations and previous observations for analogous An(IV) and Zr(IV) systems. The formation and stability of these hitherto unknown Tc(IV) species are supported by DFT calculations. Based on the newly generated experimental data and previous spectroscopic observations, new comprehensive chemical, thermodynamic and activity models (SIT, Pitzer) for these systems are derived.

 Please wait while we load your content...
Please wait while we load your content...