Coordination of Zn2+ and Cu2+ to the membrane disrupting fragment of amylin†
Abstract
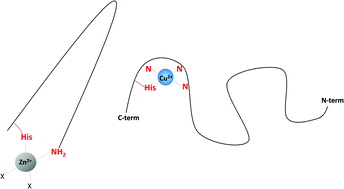
Amylin, a small peptide co-secreted from pancreatic β-cells together with insulin, is one of the hallmarks of type II diabetes. In the course of this disease, it misfolds into small oligomers or into an aggregated β-sheet amyloid fiber. The misfolding mechanism is not yet well understood, but it is clear that metal ions such as zinc and copper play an important role in the process. In this work, the coordination chemistry of Zn2+ and Cu2+ with the membrane-disrupting part of amylin (amylin1–19) is discussed. Cu2+ alters the structure of amylin1–19 only locally, by binding to His18 imidazole and to three preceding amides at the N-terminal side of this residue. Zn2+ binds to the imidazole of His18 and the amine group of Lys1, imposing a kink in the peptide between these residues. This zinc-induced kink might be a partial explanation of the formation of prefibrillar oligomeric aggregates of amylin, which are much more toxic to β-cells than large fibrillar deposits.

 Please wait while we load your content...
Please wait while we load your content...