Kinetically “locked” metallomacrocycle†
Abstract
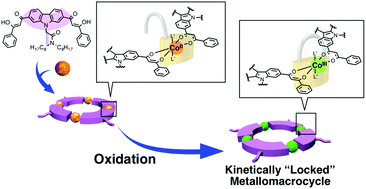
Self-assembly based on reversible metal–ligand bond formation is useful for the synthesis of discrete supramolecular nanoarchitectures. However, the architectures constructed by this technique sometimes suffer from kinetic instability due to the dissociation of metal–ligand bonds, especially under highly diluted conditions or in the presence of competitive ligands or metal ions. In this study, a kinetically stabilized metallomacrocycle was synthesized in one pot via the combination of metal-mediated self-assembly and subsequent oxidative “locking” of the coordination bonds. The macrocycle consists of four Co ions and four bis-bidentate ligands L2−. The complexation of labile Co(II) ions with the ligands afforded the macrocycle with four-fold rotational symmetry, exhibiting the right-angled geometries of the β-diketonate ligands on the carbazole. The subsequent oxidation of the Co(II) ions inside the macrocycle into Co(III) ions made the metal–ligand bonds almost inert, thus affording a kinetically locked 4 : 4 metallomacrocycle. This macrocycle showed high stability even in the presence of an excess amount of competitive ligands. X-ray crystallography of the macrocycle indicated that it assembled in a columnar manner, forming one-dimensional nanochannels in the middle of the column.

 Please wait while we load your content...
Please wait while we load your content...