The chemistry of benzodisilacyclobutenes and benzobis(disilacyclobutene)s: new development of transition-metal-catalyzed reactions, stereochemistry and theoretical studies
Abstract
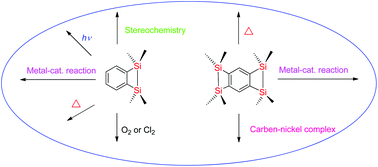
The synthesis and reactions of 3,4-benzo-1,2-disilacyclobut-3-enes and benzo[1,2:4,5]bis(1,2-disilacyclobut-3-ene)s developed by our group are reported in this review. The palladium-, platinum- and nickel-catalyzed reactions of benzodisilacyclobutenes and benzobis(disilacyclobutene)s with unsaturated compounds afford various types of products. The structures of the products are dependent upon the nature of the transition metal used as a catalyst. The reactions of cis- and trans-benzodisilacyclobutenes with alkenes and alkynes in the presence of a transition-metal catalyst proceed with high stereospecificity to give the respective adducts. The thermal reactions of cis- and trans-benzodisilacyclobutenes with various substrates also proceed stereospecifically to give adducts. The results of theoretical calculations for the platinum-catalyzed reaction of disilacyclobutene with acetylene, the nickel-catalyzed reaction of benzodisilacyclobutene and the thermal reaction of benzobis(disilacyclobutene) are discussed in this review.

 Please wait while we load your content...
Please wait while we load your content...