Sodalite-like rare-earth carbonates: a study of structural transformation and diluted magnetism†
Abstract
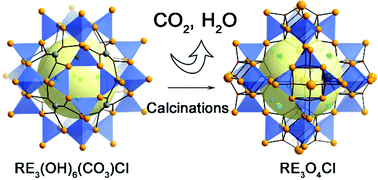
A series of novel rare earth carbonates, RE3(OH)6(CO3)Cl (RE = Dy, Er, Y), with sodalite-like (SOD-like) zeolite topologies have been successfully synthesized by introducing an appropriate amount of CO32− from NaCO3 or atmospheric carbon dioxide as a template. Single-crystal X-ray diffraction reveals that the structure consists of the RE3(OH)63+ cationic framework with a SOD-like topology built from vertex-sharing [RE4(μ3-OH)4] cubes. The CO32− anions seal the 6-ring opening and Cl− anions situated in the channels to achieve charge balance. After calcination at 370 °C, the compound RE3(OH)6(CO3)Cl in situ transforms into a new phase formulated as RE3O4Cl. Interestingly, the structure of RE3O4Cl represents a new SOD-like open framework, associated with the removal of the CO32− from RE3(OH)6(CO3)Cl. The samples are characterized by thermogravimetric analysis (TGA), elemental analysis, X-ray photoelectron spectroscopy and magnetic studies. Furthermore, single-molecule magnet behaviours can be observed for the diluted samples of Dy0.0068Y2.9932(OH)6(CO3)Cl and Dy0.0068Y2.9932O4Cl with a Dy/Y molar ratio of up to 1/440 as well as Er0.19Y2.81(OH)6(CO3)Cl with an Er/Y ratio of 1/15, showing dominant single-ion effects.

 Please wait while we load your content...
Please wait while we load your content...