Crumpled reduced graphene oxide–amine–titanium dioxide nanocomposites for simultaneous carbon dioxide adsorption and photoreduction†
Abstract
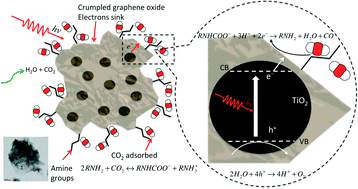
Crumpled reduced graphene oxide–amine–titanium dioxide nanocomposites (CGOATI) were synthesized by an one-step aerosol technique to enable simultaneous carbon dioxide (CO2) adsorption and photoreduction. Graphene oxide (GO), chemically modified by ethylenediamine (EDA), was crumpled using an aerosol process, encapsulating TiO2 nanoparticles to form core–shell nanostructures. The three-dimensional (3D) structure largely prevented the crumpled graphene nanosheets from restacking by minimizing π–π interactions, thus enhancing the stability of the catalyst by retaining its higher surface area. A combination of a 20% mass percentage of TiO2/GO, a 15 : 1 mass ratio of EDA/GO in precursor solution, and a 200 °C synthesis temperature led to the highest CO yield (65 μmol g−1 h−1, with an apparent quantum efficiency of 0.0094%), which was two-fold higher than that of crumpled reduced GO–TiO2 (CGOTI) and four-fold higher than that of TiO2 alone. The enhancement of CO2 photoreduction was attributed to higher CO2 adsorption on the amine-functionalized reduced-GO (r-GO) surface and the strong electron trapping capability of r-GO. The insertion of EDA on r-GO nanosheets, the adsorption of CO2 by amine groups, and the photoreduction of the adsorbed CO2 were confirmed by FTIR and XPS spectra analysis. The r-GO nanosheets themselves were simultaneously photoreduced during CO2 photoreduction. Raman spectroscopy and conductivity measurements showed that photoreduced r-GO had a higher electronic conductivity than thermally reduced r-GO, and led to more effective CO2 photoreduction. This study offers new insights into the design and fabrication of graphene-based photocatalysts for CO2 photoreduction.


 Please wait while we load your content...
Please wait while we load your content...