Fe-doped Beta zeolite from organotemplate-free synthesis for NH3-SCR of NOx†
Abstract
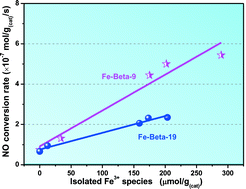
Two types of Beta zeolites, one from organotemplate-free synthesis with a Si/Al ratio of 9 and the other from a commercial one with a Si/Al ratio of 19, were employed here to dope Fe for NH3-SCR of NOx. Fe-Beta (Si/Al = 9) exhibits much higher activity than Fe-Beta (Si/Al = 19), especially at low-temperature regions (<250 °C). In addition, it also exhibits better hydrothermal stability as compared with Fe-Beta (Si/Al = 19), which demonstrates that it is a promising SCR catalyst with good activity as well as hydrothermal stability. The correlation between the quantitative calculation of the content of isolated Fe3+ in Beta zeolites and the NO conversion rate at 150 °C shows a linear relationship, suggesting that the isolated Fe3+ species affect the SCR activity directly. The higher activity of the Fe-Beta-9 catalyst is supposed to be related not only to the isolated Fe3+ but also to the acidity. Furthermore, the template-free synthesized Beta zeolite shows less dealumination during hydrothermal aging and therefore better hydrothermal stability during the SCR reaction.

 Please wait while we load your content...
Please wait while we load your content...