Encapsulation of chiral Fe(salen) in mesoporous silica structures for use as catalysts to produce optically active sulfoxides†
Abstract
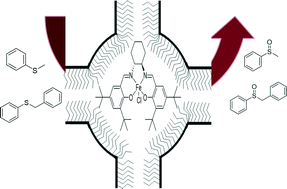
Solid catalysts which are heterogeneous at the macroscopic scale but homogeneous at the microscopic level were prepared by the encapsulation of Fe(salen) by a “ship in a bottle” approach. This approach permits the synthesis of a “free” Fe(salen) complex inside the nanocages of SBA-16 and m-MCF, having conformational freedom and behaving as a complex in solution. These materials were used as catalysts for asymmetric oxidation of sulfides. The entrance sizes of the mesoporous materials SBA-16 and m-MCF were tuned by changing the synthesis parameters and by silylation of the silica surface with n-propyl groups, which resulted in materials with different Fe(salen) loadings. Chiral Fe(salen) trapped in m-MCF materials showed higher activity than the complex immobilized on SBA-16. The activity and enantioselectivity of the catalysts based on m-MCF were on a par with the homogeneous counterpart under specific conditions. The heterogenized catalysts presented a limited recyclability; however, they were clearly advantageous compared to the homogenous counterpart, where reutilization was not possible.

 Please wait while we load your content...
Please wait while we load your content...