Continuous ruthenium-catalyzed methoxycarbonylation with supercritical carbon dioxide
Abstract
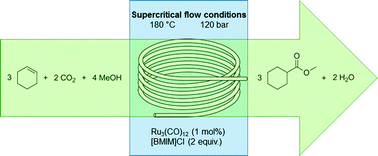
The methoxycarbonylation of cyclohexene with carbon dioxide over a ruthenium catalyst was realized in a micro flow system under supercritical conditions. Instead of the toxic and flammable carbon monoxide, this process utilizes carbon dioxide, thereby avoiding issues with bulk transportation of carbon monoxide as well as eliminating the need for safety precautions associated with the use of carbon monoxide. Obtained was a 77% yield of the ester product at 180 °C, 120 bar and with a 90 min residence time, which is over five times faster than for the same reaction performed under subcritical conditions in batch. An important factor for the performance of the system was to have a sufficiently polar supercritical mixture, allowing the catalyst to dissolve well. The optimal temperature for the reaction was 180 °C, as the activity of the system dropped considerably at higher temperatures, most likely due to catalyst deactivation.
- This article is part of the themed collection: Catalysis in Flow Chemistry

 Please wait while we load your content...
Please wait while we load your content...