Extensive H-atom abstraction from benzoate by OH-radicals at the air–water interface†
Abstract
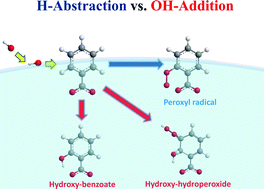
Much is known about OH-radical chemistry in the gas-phase and bulk water. Important atmospheric and biological processes, however, involve little investigated OH-radical reactions at aqueous interfaces with hydrophobic media. Here, we report the online mass-specific identification of the products and intermediates generated on the surface of aqueous (H2O, D2O) benzoate-h5 and -d5 microjets by ∼8 ns ˙OH(g) pulses in air at 1 atm. Isotopic labeling lets us unambiguously identify the phenylperoxyl radicals that ensue H-abstraction from the aromatic ring and establish a lower bound (>26%) to this process as it takes place in the interfacial water nanolayers probed by our experiments. The significant extent of H-abstraction vs. its negligible contribution both in the gas-phase and bulk water underscores the unique properties of the air–water interface as a reaction medium. The enhancement of H-atom abstraction in interfacial water is ascribed, in part, to the relative destabilization of a more polar transition state for OH-radical addition vs. H-abstraction due to incomplete hydration at the low water densities prevalent therein.

 Please wait while we load your content...
Please wait while we load your content...