Theoretical and experimental investigations of rate coefficients of O(1D) + CH4 at low temperature
Abstract
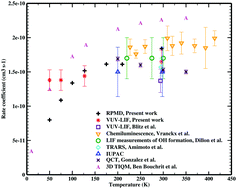
The rate coefficients of the barrierless O(1D) + CH4 reaction are determined both theoretically and experimentally at 50–296 K. For the calculations, ring polymer molecular dynamics (RPMD) simulations are performed on the basis of a new neural network potential energy surface (PES) in the reactant asymptotic part. Only the reactant asymptotic part of the PES is constructed because of its barrierless and exothermic properties. Experimentally, the reaction rate coefficients are measured using a supersonic flow reactor. Pulsed laser photolysis of O3 molecules is used as the source of O(1D) atoms, which are detected directly through vacuum ultraviolet laser induced fluorescence at 115 nm. The branching ratio for H atom production is measured by comparing the H atom yields of the O(1D) + CH4 and O(1D) + H2 reactions. At T ≥ 75 K, good agreement between theoretical and experimental rate coefficients is found, while at 50 K, the larger difference is discussed in detail.

 Please wait while we load your content...
Please wait while we load your content...