Multiple-decker and ring sandwich formation of manganese–benzene organometallic cluster anions: MnnBzn− (n = 1–5 and 18)†
Abstract
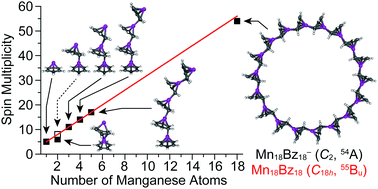
Organometallic multiple-decker sandwich clusters are topics of great interest due to their unique electronic and magnetic properties originating from anisotropic structures. We report a joint anion photoelectron spectroscopic and computational study on a new family of manganese (Mn)–benzene (Bz) anionic clusters MnnBzn−. In stark contrast to the most widely studied vanadium–Bz sandwich clusters, it is found that MnnBzn− (n = 1–5) clusters exhibit unprecedented multiple-decker structures with a tilted Mn–Bz stacking and a monotonically increasing behavior of their high spin multiplicities. Furthermore, a couple of closed ring forms of Mn18Bz18− and its neutral state are computationally anticipated as an intriguing “cluster of Mn1Bz1 clusters” in which the neutral Mn18Bz18 has extremely high C18h symmetry with an uncommon spin state of 2S + 1 = 55. The extensively delocalized electron environment of Mn18Bz18 allows the simple Hückel model to reveal the strong intra-atomic exchange interactions within the Mn 3d electrons.

 Please wait while we load your content...
Please wait while we load your content...