Anomalous pressure effects on the photoreaction of a light-sensor protein from Synechocystis, PixD (Slr1694), and the compressibility change of its intermediates†
Abstract
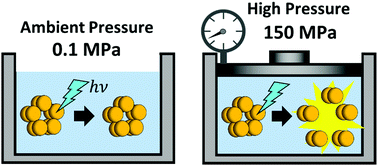
SyPixD (Slr1694) is a blue-light receptor that contains a BLUF (blue-light sensor using a flavin chromophore) domain for the function of phototaxis. The key reaction of this protein is a light-induced conformational change and subsequent dissociation reaction from the decamer to the dimer. In this study, anomalous effects of pressure on this reaction were discovered, and changes in the compressibility of its short-lived intermediates were investigated. While the absorption spectra of the dark and light states are not sensitive to pressure, the formation yield of the first intermediate decreases with pressure to about 40% at 150 MPa. Upon blue-light illumination with a sufficiently strong intensity, the transient grating signal, which represents the dissociation of the SyPixD decamer, was observed at 0.1 MPa, and the signal intensity significantly decreased with increasing pressure. This behavior shows that the dissociation of the decamer from the second intermediate state is suppressed by pressure. However, while the decamer undergoes no dissociation upon excitation of one monomer unit at 0.1 MPa, dissociation is gradually induced with increasing pressure. For solving this strange behavior, the compressibility changes of the intermediates were measured as a function of pressure at weak light intensity. Interestingly, the compressibility change was negative at low pressure, but became positive with increasing pressure. Because the compressibility is related to the volume fluctuation, this observation suggests that the driving force for this reaction is fluctuation of the protein. The relationship between the cavities at the interfaces of the monomer units and the reactivity was also discussed.

 Please wait while we load your content...
Please wait while we load your content...