Unraveling the hydration-induced ground-state change of AtO+ by relativistic and multiconfigurational wave-function-based methods†
Abstract
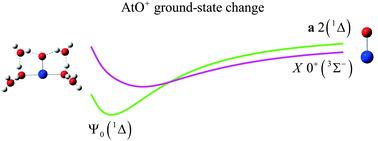
The AtO+ cation is one of the main chemical forms that appear in the astatine Pourbaix diagram. This form can react with closed-shell species in solution, while in the gas phase, it has a spin-triplet ground spin–orbit-free (SOF) state. Spin–orbit coupling (SOC) mixes its MS = 0 component with the 1Σ+ singlet-spin component, while keeping an essentially-spin-triplet SOC ground-state. Therefore, it was suggested that AtO+ undergoes a hydration-induced ground-state change to explain its reactivity in solution with closed-shell species [J. Phys. Chem. B, 2013, 117, 5206–5211]. In this work, we track the nature of the low-lying SOF and SOC states when the hydration sphere of AtO+ is stepwise increased, using relativistic and multiconfigurational wave-function-based methods. This work clarifies previous studies by (i) giving additional arguments justifying a solvation-induced ground-state change in this system and (ii) clearly identifying for the first time the nature of the involved SOF and SOC many-electron states. Indeed, we find at the SOF level that AtO+ undergoes a ground-state reversal between 3Σ− and the closed-shell component of 1Δ, which leads to an essentially-spin-singlet and closed-shell SOC ground-state. This explains the observed reactivity of AtO+ with closed-shell species in solution.


 Please wait while we load your content...
Please wait while we load your content...