Radiative cooling of H3O+ and its deuterated isotopologues†
Abstract
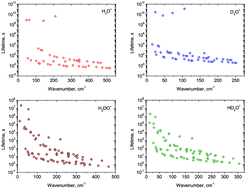
In conjunction with ab initio potential energy and dipole moment surfaces for the electronic ground state, we have made a theoretical study of the radiative lifetimes for the hydronium ion H3O+ and its deuterated isotopologues. We compute the ro-vibrational energy levels and their associated wavefunctions together with Einstein coefficients for electric dipole transitions. A detailed analysis of the stability of the ro-vibrational states has been carried out and the longest-living states of the hydronium ions have been identified. We report estimated radiative lifetimes and cooling functions for temperatures <200 K. A number of long-living meta-stable states are identified, capable of population trapping.

 Please wait while we load your content...
Please wait while we load your content...