Quantitative determination of activation energies in mechanochemical reactions†
Abstract
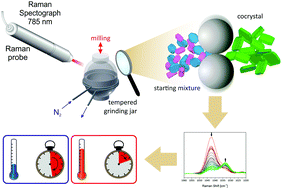
Mechanochemical reactions often result in 100% yields of single products, making purifying procedures obsolete. Mechanochemistry is also a sustainable and eco-friendly method. The ever increasing interest in this method is contrasted by a lack in mechanistic understanding of the mechanochemical reactivity and selectivity. Recent in situ investigations provided direct insight into formation pathways. However, the currently available theories do not predict temperature T as an influential factor. Here, we report the first determination of an apparent activation energy for a mechanochemical reaction. In a temperature-dependent in situ study the cocrystallisation of ibuprofen and nicotinamide was investigated as a model system. These experiments provide a pivotal step towards a comprehensive understanding of milling reaction mechanisms.

 Please wait while we load your content...
Please wait while we load your content...