Influence of counterions on the conformation of conjugated polyelectrolytes: the case of poly(thiophen-3-ylacetic acid)†
Abstract
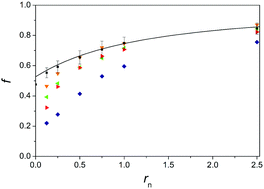
The addition of simple salt to a solution of conjugated polyelectrolyte can lead to substantial changes in its optical properties caused by the conformational change of the polymer chain. The effect of the addition of alkali metal and tetraalkylammonium chlorides to solutions of lithium salt of poly(thiophen-3-ylacetic acid) is investigated by NMR. The fractions of free alkali metal counterions are in agreement with predictions of the cylindrical Poisson–Boltzmann cell model. On the other hand, the fractions of free tetraalkylammonium counterions deviate from the prediction of this model and diminish with increasing size of these counterions. This trend is consistent with observed ultraviolet-visible absorption spectra and measured self-diffusion coefficients of the polyion in mixtures with tetraalkylammonium salts. A transition to more constricted conformation of the polyion chain becomes more pronounced with the lengthening of alkyl groups in the added tetraalkylammonium cation. Taking into account the obtained fractions of free counterions, existing thermodynamic data are reanalysed in order to determine thermodynamic parameters for binding of different counterions to the polyion. This analysis shows that standard enthalpies of binding of alkali metal counterions are distinctively different, which is most probably related to differences in hydration shells of counterions. On the other hand, such an analysis fails in the case of tetraalkylammonium chlorides where obviously more complex changes take place.

 Please wait while we load your content...
Please wait while we load your content...