The underlying mechanisms for self-healing of poly(disulfide)s
Abstract
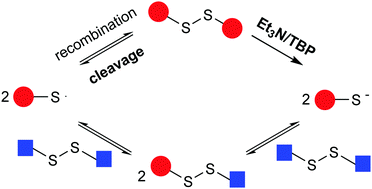
Recently, self-healing polymers based on disulfide compounds have gained attention due to the versatile chemistry of disulfide bonds and easy implementation into polymeric materials. However, the underlying mechanisms of disulfide exchange which induce the self-healing effect in poly(disulfide)s remain unclear. In this work, we elucidate the process of disulfide exchange using a variety of spectroscopic techniques. Comparing a model exchange reaction of 4-aminophenyl disulfide and diphenyl disulfide with modified reactions in the presence of additional radical traps or radical sources confirmed that the exchange reaction between disulfide compounds occurred via a radical-mediated mechanism. Furthermore, when investigating the effect of catalysts on the model exchange reaction, it could be concluded that catalysts enhance the disulfide exchange reaction through the formation of S-based anions in addition to the radical-mediated mechanism.


 Please wait while we load your content...
Please wait while we load your content...