Deciphering the cryptic role of a catalytic electron in a photochemical bond dissociation using excited state aromaticity markers†
Abstract
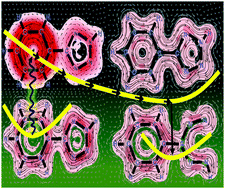
The breaking of bonds by catalytic electrons has gained prominence very recently but has been limited to cases where electrons from external sources have been used. Here, we show that upon photoexcitation, an electron of intramolecular origin is transferred from one part of a molecule to another followed by bond cleavage and then returns to its original moiety on completion of its catalytic function. By a proper assessment of the dramatic changes in aromaticity in excited-state intermediates along the photoreaction coordinate captured by the magnetically induced current density (MICD) technique, we show that in 5-phenyltetrazole, an excited electron, which migrates from the phenyl ring to the tetrazole ring, induces bond cleavage catalytically. Using the MICD technique, we establish for the first time a link between the phenomenon of excited-state electron/charge transfer among aromatic rings and the intricate interplay of aromatic, antiaromatic and non-aromatic states.

 Please wait while we load your content...
Please wait while we load your content...