Fluorescence lifetime imaging of optically levitated aerosol: a technique to quantitatively map the viscosity of suspended aerosol particles†
Abstract
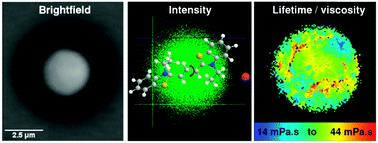
We describe a technique to measure the viscosity of stably levitated single micron-sized aerosol particles. Particle levitation allows the aerosol phase to be probed in the absence of potentially artefact-causing surfaces. To achieve this feat, we combined two laser based techniques: optical trapping for aerosol particle levitation, using a counter-propagating laser beam configuration, and fluorescent lifetime imaging microscopy (FLIM) of molecular rotors for the measurement of viscosity within the particle. Unlike other techniques used to measure aerosol particle viscosity, this allows for the non-destructive probing of viscosity of aerosol particles without interference from surfaces. The well-described viscosity of sucrose aerosol, under a range of relative humidity conditions, is used to validate the technique. Furthermore we investigate a pharmaceutically-relevant mixture of sodium chloride and salbutamol sulphate under humidities representative of in vivo drug inhalation. Finally, we provide a methodology for incorporating molecular rotors into already levitated particles, thereby making the FLIM/optical trapping technique applicable to real world aerosol systems, such as atmospheric aerosols and those generated by pharmaceutical inhalers.


 Please wait while we load your content...
Please wait while we load your content...