First-principles assessment of CO2 capture mechanisms in aqueous piperazine solution†
Abstract
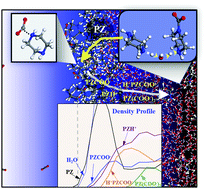
Piperazine (PZ) and its blends have emerged as attractive solvents for CO2 capture, but the underlying reaction mechanisms still remain uncertain. Our study particularly focuses on assessing the relative roles of PZCOO− and PZH+ produced from the PZ + CO2 reaction. PZCOO− is found to directly react with CO2 forming COO−PZCOO−, whereas PZH+ will not. However, COO−PZCOO− appears very unlikely to be produced in thermodynamic equilibrium with monocarbamates, suggesting that its existence would predominantly originate from the surface reaction that likely occurs. We also find production of H+PZCOO− to be more probable with increasing CO2 loading, due partly to the thermodynamic favorability of the PZH+ + PZCOO− → H+PZCOO− + PZ reaction; the facile PZ liberation may contribute to its relatively high CO2 absorption rate. This study highlights an accurate description of surface reaction and the solvent composition effect is critical in thermodynamic and kinetic models for predicting the CO2 capture processes.

 Please wait while we load your content...
Please wait while we load your content...