Is formamide a geochemically plausible prebiotic solvent?†
Abstract
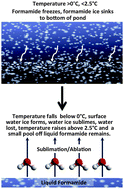
From a geochemical perspective, significant amounts of pure formamide (HCONH2) would have likely been rare on the early Earth. There may have been mixed formamide–water solutions, but even in the presence of catalyst, solutions with >20 weight% water in formamide would not have produced significant amounts of prebiotic compounds. It might be feasible to produce relatively pure formamide by a rare occurrence of freezing formamide/water mixtures at temperatures lower than formamide's freezing point (2.55 °C) but greater than the freezing point of water. Because of the high density of formamide ice it would have sunk and accumulated at the bottom of the solution. If the remaining water froze on the surface of this ice, and was then removed by a sublimation–ablation process, a small amount of pure formamide ice might have been produced. In addition a recent report suggested that ∼85 weight% formamide could be prepared by a geochemical type of fractional distillation process, offering another possible route for prebiotic formamide production.
- This article is part of the themed collections: PCCP Perspectives and Prebiotic chemistry and the molecular origins of life

 Please wait while we load your content...
Please wait while we load your content...