High-pressure phase transitions in rubidium and caesium hydroxides†
Abstract
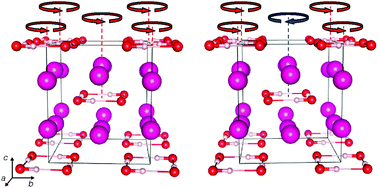
A computational investigation of the high-pressure phase sequence of the heaviest alkali hydroxides, RbOH and CsOH, shows that the phase diagram of both compounds is richer than hitherto thought. First-principles calculations suggest, based on energetics and comparisons to experimental diffraction and spectroscopy signatures, that the high-pressure phase RbOH-VI, stable above 6 GPa in experiment, should be assigned the KOH-VI structure type, and features localised hydrogen-bonded (OH)4 units. Meanwhile, a new high-pressure phase CsOH-VII is predicted to be stable above 10 GPa in an isosymmetric phase transition that, like RbOH-VI, marks the transition from layered to three-dimensional network structures under increased compression. Both new phases highlight an unexpected flexibility of hydrogen bond network formation in a series of compounds that seemingly only vary in the cation size, and potential consequences for similar systems, such as water-carrying minerals, are discussed briefly.

 Please wait while we load your content...
Please wait while we load your content...