Why are some cyano-based ionic liquids better glucose solvents than water?†
Abstract
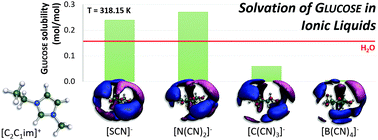
Among different classes of ionic liquids (ILs), those with cyano-based anions have been of special interest due to their low viscosity and enhanced solvation ability for a large variety of compounds. Experimental results from this work reveal that the solubility of glucose in some of these ionic liquids may be higher than in water – a well-known solvent with enhanced capacity to dissolve mono- and disaccharides. This raises questions on the ability of cyano groups to establish strong hydrogen bonds with carbohydrates and on the optimal number of cyano groups at the IL anion that maximizes the solubility of glucose. In addition to experimental solubility data, these questions are addressed in this study using a combination of density functional theory (DFT) and molecular dynamics (MD) simulations. Through the calculation of the number of hydrogen bonds, coordination numbers, energies of interaction and radial and spatial distribution functions, it was possible to explain the experimental results and to show that the ability to favorably interact with glucose is driven by the polarity of each IL anion, with the optimal anion being dicyanamide.


 Please wait while we load your content...
Please wait while we load your content...