Impact of the water dimer on the atmospheric reactivity of carbonyl oxides†
Abstract
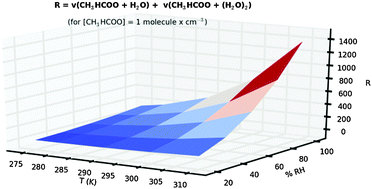
The reactions of twelve carbonyl oxides or Criegee intermediates with the water monomer and with the water dimer have been investigated employing high level theoretical methods. The study includes all possible carbonyl oxides arising from the isoprene ozonolysis and the methyl and dimethyl carbonyl oxides that originated from the reaction of ozone with several hydrocarbons. These reactions have great significance in the chemistry of the atmosphere because Criegee intermediates have recently been identified as important oxidants in the troposphere and as precursors of secondary organic aerosols. Moreover, water vapor is one of the most abundant trace gases in the atmosphere and the water dimer can trigger the atmospheric decomposition of Criegee intermediates. Our calculations show that the nature and position of the substituents in carbonyl oxides play a very important role in the reactivity of these species with both the water monomer and the water dimer. This fact results in differences in rate constants of up to six orders of magnitude depending on the carbonyl oxide. In this work we have defined an effective rate constant (keff) for the atmospheric reaction of carbonyl oxides with water vapor, which depends on the temperature and on the relative humidity as well. With this keff we show that the water dimer, despite its low tropospheric concentration, enhances the atmospheric reactivity of Criegee intermediates, but its effect changes with the nature of carbonyl oxide, ranging between 59 and 295 times in the most favorable case (syn-methyl carbonyl oxide), and between 1.4 and 3 times only in the most unfavorable case.

 Please wait while we load your content...
Please wait while we load your content...