Liquid structure of dibutyl sulfoxide
Abstract
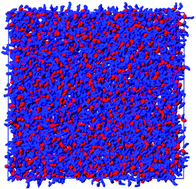
We present experimental (X-ray diffraction) data on the structure of liquid dibutyl sulfoxide at 320 K and rationalise the data by means of molecular dynamics simulations. Not unexpectedly, DBSO bearing a strong dipolar moiety and two medium length, apolar butyl chains, this compound was characterised by a distinct degree of polar vs. apolar structural differentiation at the nm spatial scale, which was fingerprinted by a low Q peak in its X-ray diffraction pattern. Similar to, but to a larger extent than its shorter chain family members (such as DMSO), DBSO was also characterised by an enhanced dipole–dipole correlation, which was responsible for a moderate Kirkwood correlation factor as well as for the self-association detected in this compound. We show, however, that the supposedly relevant hydrogen bonding correlations between oxygen and the butyl chain hydrogens are of a limited extent only, and only in the case of α-hydrogens is an appreciable indication of the existence of such an interaction found, albeit this turned out to be a mere consequence of the strong dipole–dipole correlation.

 Please wait while we load your content...
Please wait while we load your content...