Improved electron transport properties of n-type naphthalenediimide polymers through refined molecular ordering and orientation induced by processing solvents†
Abstract
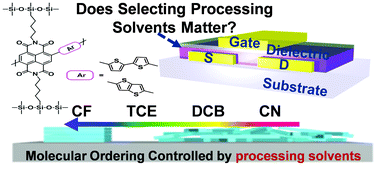
To determine the role played by the choice of processing solvents in governing the photophysics, microstructure, and charge carrier transport in naphthalenediimide (NDI)-based polymers, we have prepared two new NDI-bithiophene (T2)- and NDI-thienothiophene (TTh)-containing polymers with hybrid siloxane pentyl chains (SiC5) (P(NDI2SiC5-T2) and P(NDI2SiC5-TTh)). Among the various processing solvents studied here, the films prepared using chloroform exhibited far better electron mobilities (0.16 ± 0.1–0.21 ± 0.05 cm2 V−1 s−1) than the corresponding samples prepared from different solvents, exceeding one order of magnitude higher, indicating the significant influence of the processing solvent on the charge transport. Upon thin-film analysis using atomic force microscopy and grazing incidence X-ray diffraction, we discovered that molecular ordering and orientation are affected by the choice of the processing solvent, which is responsible for the change in the transport characteristics of this class of polymers.

 Please wait while we load your content...
Please wait while we load your content...