Determination of Kamlet–Taft parameters for selected solvate ionic liquids†
Abstract
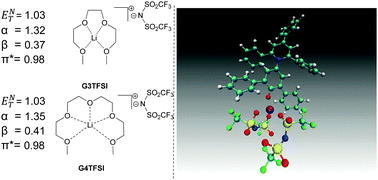
The normalised polarity ENT and Kamlet–Taft parameters of recently described solvate ionic liquids, composed of lithium bis(trifluoromethyl)sulfonimide (LiTFSI) in tri- (G3TFSI) or tetraglyme (G4TFSI) have been determined and compared to the parent glyme (G3 and G4). We show that these solvate ionic liquids have a high polarity (G3TFSI, (ENT) = 1.03; G4TFSI, (ENT) = 1.03) and display very high electron pair accepting characteristics (G3TFSI, α = 1.32; G4TFSI, α = 1.35). Molecular dynamics simulations suggest that the chelated lithium cation is responsible for this observation. The relatively small hydrogen bond acceptor (β) values for these systems (G3TFSI, β = 0.41; G4TFSI, β = 0.37) are thought to be due primarily to the TFSI anion, which is supplemented slightly by the glyme oxygen atom. In addition, these solvate ionic liquids are found to have a high polarisability (G3TFSI, π* = 0.94; G4TFSI, π* = 0.90).

 Please wait while we load your content...
Please wait while we load your content...