Hydrogen adsorption on two catalysts for the ortho- to parahydrogen conversion: Cr-doped silica and ferric oxide gel†
Abstract
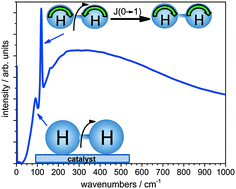
Molecular hydrogen exists in two spin-rotation coupled states: parahydrogen and orthohydrogen. Due to the variation of energy with rotational level, the occupation of ortho- and parahydrogen states is temperature dependent, with parahydrogen being the dominant species at low temperatures. The equilibrium at 20 K (99.8% parahydrogen) can be reached by natural conversion only after a lengthy process. With the use of a suitable catalyst, this process can be shortened significantly. Two types of commercial catalysts currently being used for ortho- to parahydrogen conversion are: iron(III) oxide (Fe2O3, IONEX®), and chromium(II) oxide doped silica catalyst (CrO·SiO2, OXISORB®). We investigate the interaction of ortho- and parahydrogen with the surfaces of these ortho–para conversion catalysts using neutron vibrational spectroscopy. The catalytic surfaces have been characterized using X-ray absorption fine structure (XAFS) and X-ray/neutron pair distribution function measurements.
- This article is part of the themed collection: Neutron Scattering in Catalysis and Energy Materials

 Please wait while we load your content...
Please wait while we load your content...