Frozen-density embedding theory with average solvent charge densities from explicit atomistic simulations
Abstract
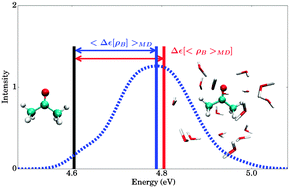
Besides molecular electron densities obtained within the Born–Oppenheimer approximation (ρB(r)) to represent the environment, the ensemble averaged density (〈ρB〉(r)) is also admissible in frozen-density embedding theory (FDET) [Wesolowski, Phys. Rev. A, 2008, 77, 11444]. This makes it possible to introduce an approximation in the evaluation of the solvent effect on quantum mechanical observables consisting of replacing the ensemble averaged observable by the observable evaluated at ensemble averaged ρB(r). This approximation is shown to affect negligibly the solvatochromic shift in the absorption of hydrated acetone. The proposed model provides a continuum type of representation of the solvent, which reflects nevertheless its local structure, and it is to be applied as a post-simulation analysis tool in atomistic level simulations.
- This article is part of the themed collection: Developments in Density Functional Theory

 Please wait while we load your content...
Please wait while we load your content...