Enhanced anion binding by heteroatom replacement in bambusurils†
Abstract
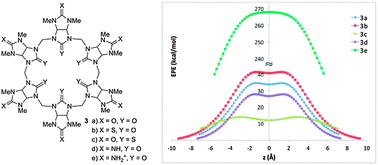
This study was driven by the hypothesis that heteroatom replacement in bambusurils could significantly modify their anion binding properties. Indeed, calculations with various glycoluril and bambusuril analogs predict that such replacements significantly alter their molecular electrostatic potential and binding properties. Both polarization and electrostatic interactions contribute to anion binding, leading to a general trend of affinity among the neutral molecules: X = S > O > NH. In bambusurils the heteroatom replacement at the portal carbonyls affect the induced dipole more significantly than replacements at the equatorial carbonyls. The stronger polarization and stronger anion binding manifest the increased aptitude of the portal heteroatoms as electron sinks. Notably, this study predicts that protonated aza-bambusurils would not only bind multiple anions along their main axis, but could also function as synthetic anion channels.

 Please wait while we load your content...
Please wait while we load your content...