A systematic study on Pt based, subnanometer-sized alloy cluster catalysts for alkane dehydrogenation: effects of intermetallic interaction†
Abstract
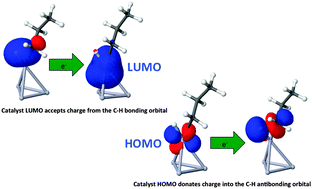
Platinum-based bimetallic nanoparticles are analyzed by the application of density functional theory to a series of tetrahedral Pt3X cluster models, with element X taken from the P-block, preferably group 14, or from the D-block around group 10. Almost identical cluster geometries allow a systematic investigation of electronic effects induced by different elements X. Choosing the propane-to-propene conversion as the desired dehydrogenation reaction, we provide estimates for the activity and selectivity of the various catalysts based on transition state theory. No significant Brønsted–Evans–Polanyi-relation could be found for the given reaction. A new descriptor, derived from an energy decomposition analysis, captures the effect of element X on the rate-determining step of the first hydrogen abstraction. Higher activities than obtained for pure Pt4 clusters are predicted for Pt alloys containing Ir, Sn, Ge and Si, with Pt3Ir showing particularly high selectivity.

 Please wait while we load your content...
Please wait while we load your content...