Photo-degradation in air of the active layer components in a thiophene–quinoxaline copolymer:fullerene solar cell†
Abstract
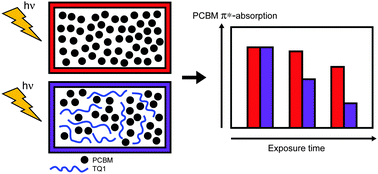
We have studied the photo-degradation in air of a blend of [6,6]-phenyl-C61-butyric acid methyl ester (PCBM) and poly[2,3-bis-(3-octyloxyphenyl)quinoxaline-5,8-diyl-alt-thiophene-2,5-diyl] (TQ1), and how the photo-degradation affects the solar cell performance. Using near-edge X-ray absorption fine structure (NEXAFS) spectroscopy, changes to the electronic structure of TQ1 and PCBM caused by illumination in ambient air are investigated and compared between the pristine materials and the blend. The NEXAFS spectra show that the unoccupied molecular orbitals of TQ1 are not significantly changed by the exposure of pristine TQ1 to light in air, whereas those of PCBM are severely affected as a result of photo-induced degradation of PCBM. Furthermore, the photo-degradation of PCBM is accelerated by blending it with TQ1. While the NEXAFS spectrum of TQ1 remains unchanged upon illumination in air, its valence band spectrum shows that the occupied molecular orbitals are weakly affected. Yet, UV-Vis absorption spectra demonstrate photo-bleaching of TQ1, which is attenuated in the presence of PCBM in blend films. Illumination of the active layer of TQ1:PCBM solar cells prior to cathode deposition causes severe losses in electrical performance.

 Please wait while we load your content...
Please wait while we load your content...