Mechanistic and microkinetic analysis of CO2 hydrogenation on ceria†
Abstract
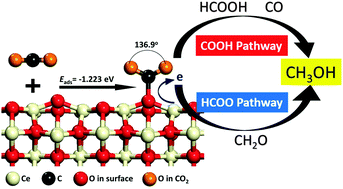
We use density functional theory (DFT) calculations to investigate the mechanism of CO2 hydrogenation to methanol on a reduced ceria (110) catalyst, which has previously been shown to activate CO2. Two reaction channels to methanol are identified: (1) COOH pathway via a carboxyl intermediate and (2) HCOO pathway via a formate intermediate. While formaldehyde (H2CO) appears to be the key intermediate for methanol synthesis, other intermediates, including carbine diol, formic acid and methanol, are not feasible due to their high formation energies. Furthermore, direct formyl hydrogenation to formaldehyde is not feasible due to its high activation barrier. Instead, we find that conversion of H-formalin (H2COOH*) to formaldehyde is kinetically more favorable. The formaldehyde is then converted to methoxy (H3CO*), and finally hydrogenated to form methanol. Microkinetic analyses reveal the rate-limiting steps in the reaction network and establish that the HCOO route is the dominant pathway for methanol formation on this catalyst.

 Please wait while we load your content...
Please wait while we load your content...