HSO3Cl: a prototype molecule for studying OH-stretching overtone induced photodissociation†
Abstract
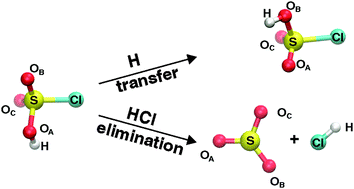
Vibrationally induced photodissociation in sulfurochloridic acid (HSO3Cl) is found to be a viable process to form SO3 and HCl from excitations of the OH-stretching overtone starting at νOH = 4. Reactive molecular dynamics simulations on a fully-dimensional potential energy surface fitted to MP2 calculations show that hydrogen transfer and HCl elimination compete with one another on the nanosecond time scale. Excitation with 5 and 6 quanta in the OH-stretch direct elimination of HCl is a dominant process on the several hundred picosecond time scale. At longer times, HCl formation is preceded by intramolecular hydrogen transfer and concomitant excitation of torsional degrees of freedom. As HSO3Cl is a suitable proxy for H2SO4, which is relevant for weather and climate in the upper atmosphere, it is concluded that vibrationally induced photodissociation is a possible mechanism for H2SO4 decomposition. Final state energy distributions for different internal degrees of freedom are predicted which should be observable in laboratory measurements.


 Please wait while we load your content...
Please wait while we load your content...