Access to aliphatic protons as reporters in non-deuterated proteins by solid-state NMR†
Abstract
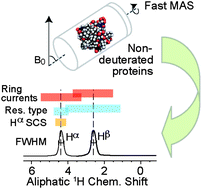
Interactions within proteins, with their surrounding, and with other molecules are mediated mostly by hydrogen atoms. In fully protonated, inhomogeneous, or larger proteins, however, aliphatic proton shifts tend to show little dispersion despite fast Magic-Angle Spinning. 3D correlations dispersing aliphatic proton shifts by their better resolved amide N/H shifts can alleviate this problem. Using inverse second-order cross-polarization (iSOCP), we here introduce dedicated and improved means to sensitively link site-specific chemical shift information from aliphatic protons with a backbone amide resolution. Thus, even in cases where protein deuteration is impossible, this approach may enable access to various aspects of protein functions that are reported on by protons.

 Please wait while we load your content...
Please wait while we load your content...