The determining factor for interstitial oxygen formation in Ruddlesden–Popper type La2NiO4-based oxides†
Abstract
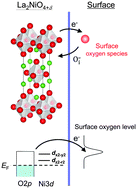
The interstitial oxygen formation mechanism in La2NiO4-based oxides was studied using soft X-ray absorption spectroscopy. When the interstitial oxygen concentration increased, the pre-edge peak of O K-edge spectra increased while Ni L-edge spectra was almost invariant. These spectral changes strongly suggest the significant contribution of ligand oxygen to interstitial oxygen formation by providing/accepting electronic charge carriers. The variation of the integrated peak intensity of the O K-edge strongly suggests that interstitial oxygen formation is determined by the equilibrium unoccupied pDOS of ligand oxygen. From this hypothesis, we propose that modulating the electronic structure is the key to control the capability of interstitial oxygen formation in La2NiO4-based oxides.

 Please wait while we load your content...
Please wait while we load your content...