Role of electrostatic interactions in the adsorption kinetics of nanoparticles at fluid–fluid interfaces†
Abstract
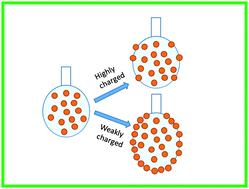
The adsorption of particles to the fluid–fluid interface is a key factor for the stabilization of fluid–fluid interfaces such as those found in emulsions, foams and bijels. However, for the formation of stable particle–laden interfaces, the particles must migrate to the interface from the bulk. Recent studies show that the adsorption of particles to the interface formed during emulsification is influenced by the surface charge of the particles. To further investigate this phenomenon, we study the effect of the surface charge of the particle on the adsorption kinetics of particles to the oil–water interface. By suspending a drop of aqueous dispersion of charge stabilized nanoparticles in decane, the adsorption dynamics of particles to the decane–water interface is studied using the dynamic surface tension measurements. When the particles are highly charged (low salt), a negligible change in the interface tension is observed indicating that almost no particles are adsorbed. These results show that the charged particles experience an energy barrier when they approach the interface. But when the particle surface charge is screened by the addition of monovalent salt, a significant reduction in surface tension is observed indicating the migration and adsorption of particles to the decane–water interface. We estimate the effective diffusivity of particles to the interface by analyzing the initial decay in the measured surface tension by considering particle laden drops containing different amounts of salt using the modified Ward and Tordai theory. This effective diffusivity is used to calculate the energy barrier for the adsorption of particles to the interface. The energy barrier from the analysis of dynamic surface tension data agrees well with the concept of image charge repulsion which inhibits the adsorption of highly charged particles to the interface. By considering various types of relevant interactions, we derive an analytical expression that qualitatively captures the effect of the surface charge on the equilibrium surface coverage of particles at the drop surface.

 Please wait while we load your content...
Please wait while we load your content...