LiCl solvation in N-methyl-acetamide (NMA) as a model for understanding Li+ binding to an amide plane†
Abstract
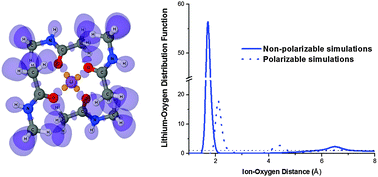
The thermodynamics of ion solvation in non-aqueous solvents remains of great significance for understanding cellular transport and ion homeostasis for the design of novel ion-selective materials and applications in molecular pharmacology. Molecular simulations play pivotal roles in connecting experimental measurements to the microscopic structures of liquids. One of the most useful and versatile mimetic systems for understanding biological ion transport is N-methyl-acetamide (NMA). A plethora of theoretical studies for ion solvation in NMA have appeared recently, but further progress is limited by two factors. One is an apparent lack of experimental data on solubility and thermodynamics of solvation for a broad panel of 1 : 1 salts over an appropriate temperature and concentration range. The second concern is more substantial and has to do with the limitations hardwired in the additive (fixed charge) approximations used for most of the existing force-fields. In this submission, we report on the experimental evaluation of LiCl solvation in NMA over a broad range of concentrations and temperatures and compare the results with those of MD simulations with several additive and one polarizable force-field (Drude). By comparing our simulations and experimental results to density functional theory computations, we discuss the limiting factors in existing potential functions. To evaluate the possible implications of explicit and implicit polarizability treatments on ion permeation across biological channels, we performed potential of mean force (PMF) computations for Li+ transport through a model narrow ion channel with additive and polarizable force-fields.

 Please wait while we load your content...
Please wait while we load your content...