Conformational ensemble of human α-synuclein physiological form predicted by molecular simulations†
Abstract
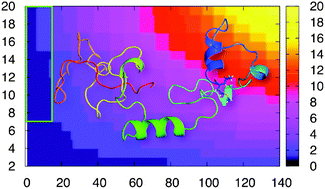
We perform here enhanced sampling simulations of N-terminally acetylated human α-synuclein, an intrinsically disordered protein involved in Parkinson's disease. The calculations, consistent with experiments, suggest that the post-translational modification leads to the formation of a transient amphipathic α-helix. The latter, absent in the non-physiological form, alters protein dynamics at the N-terminal and intramolecular interactions.
- This article is part of the themed collection: Exploring the conformational heterogeneity of biomolecules: theory and experiments

 Please wait while we load your content...
Please wait while we load your content...