Crystallization induced green-yellow-orange emitters based on benzoylpyrenes†
Abstract
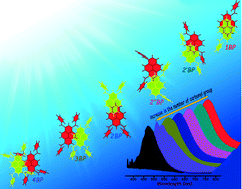
We report the synthesis, X-ray structures and photophysical properties of a few benzoylpyrene (BP) derivatives. Steric hindrance due to incremental benzoyl groups causes a systematic reduction in the orbital overlap (π–π) between vicinal pyrene units affording green-yellow-orange solid-state emitters. Crystallization induced emission could arise from: i) electronic (dipolar/excitonic) interactions, ii) arrested bond rotations, and/or iii) lack of solvation in crystalline 1–4BP (ΦFl ∼ 2–26%) when compared to that in solution (ΦFl ≤ 1%). Our earlier effort [Chem. Commun. 2014, 50, 8644] on progressive acylation, in contrast to benzoylation, results in a gradual increase in the π–π overlap between vicinal pyrenes.

 Please wait while we load your content...
Please wait while we load your content...