A facile strategy for accessing 3-alkynylchromones through gold-catalyzed alkynylation/cyclization of o-hydroxyarylenaminones†
Abstract
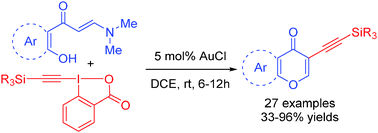
A strategy based on tandem alkynylation of o-hydroxyarylenaminones followed by intramolecular cyclization has been developed to generate a diverse array of 3-alkynyl chromones. The functionality embedded in these key intermediates enables their facile elaboration into more diverse structures by a variety of functionalizations and ring-forming processes.

 Please wait while we load your content...
Please wait while we load your content...